14+ ge orbital diagram
The first number is the principal quantum number n and the letter represents the value of l angular momentum. Start these orbital diagrams from the 1s orbital filled with two opposite-facing arrows to represent the two electrons in a different spin.

Polyoxocationic Antimony Oxide Cluster With Acidic Protons Science Advances
1 Draw the orbital diagrams for the following atomsions.
. Fe 3e Fe 3. Electron configurations have the format. The orbital diagram will be filled in the same order as described by the Aufbau principle.
Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. There are different types of orbitals that all have different energy levels. To write the orbital diagram for the Oxygen atom O first we need to write the electron configuration for just O.
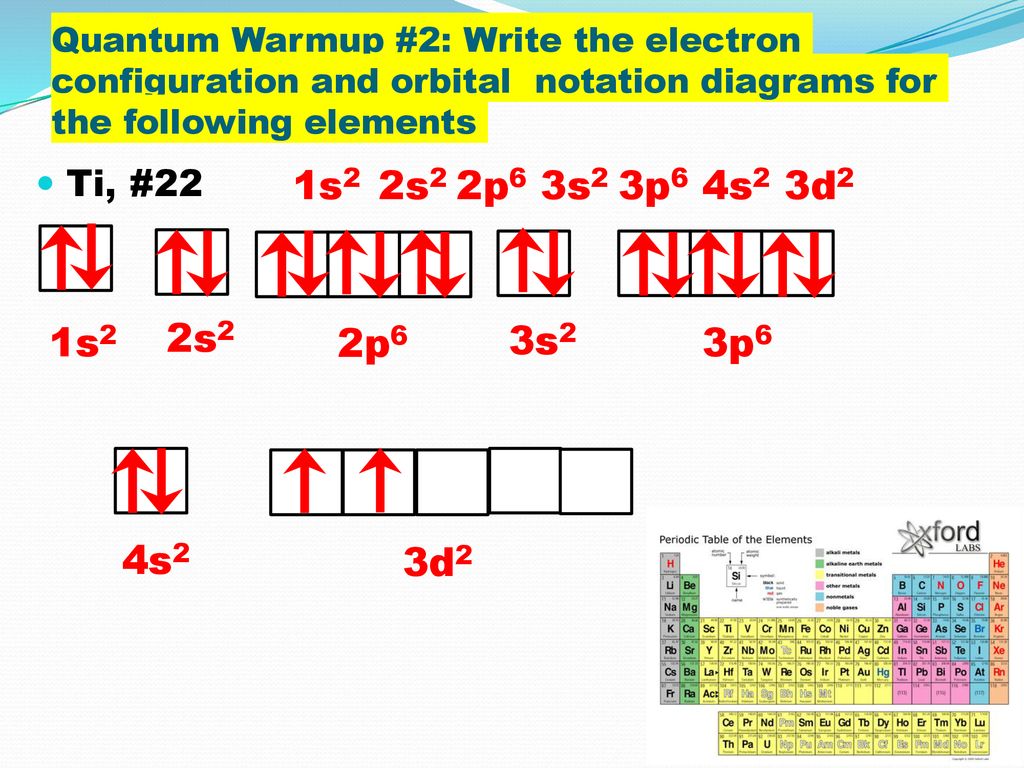
What element does the following orbital diagram represent. Going down to the next period 2. The Aufbau principle states that.
The electron configuration of iron ion Fe 3 is 1s 2 2s 2 2p 6 3s 2. Orbital diagram of Germanium. Displaying all worksheets related to - Orbital Diagram.
State the number of possible electrons described by the following quantum numbers. The electron configuration of. The electron configuration of.
Orbital diagram of Chlorine Cl 18. Worksheets are Orbital diagrams name chem work 5 5 Molecular orbital diagram key Work 14 Electron. Orbital diagram of Silicon Si 15.
1s 2s. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 2. Orbital diagram of Phosphorus P 16.
Orbital diagram of Sulfur S 17. Here the electron configuration of germanium ion Ge 2 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. Located in the IV period.
To do that we need to find the number of. Orbital diagrams can be defined as the pictorial description of electrons in an atom which is. The order in which the orbitals are filled with electrons from lower energy to higher energy is.
Electronic configuration of the. What element has the following electron. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6.
2 8 18 4. The iron atom donates two electrons in 4s orbital and an electron in 3d orbital to convert iron ion Fe 3. Ge 2e Ge 2.
Up to 24 cash back 2. F orbital contains 7 boxes that can hold a maximum of 14 electrons. 532 gcm 3.
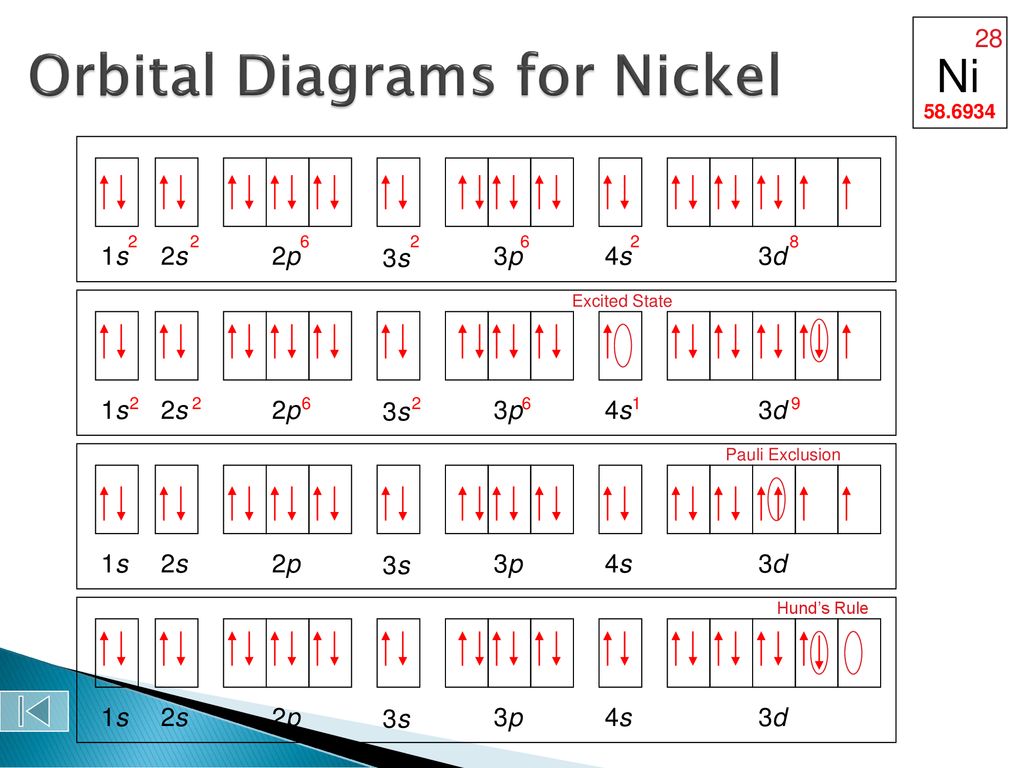
Orbital Diagram of All Elements Diagrams. Ge Germanium is an element with position number 32 in the periodic table. These orbitals are filled with electrons the amount of electrons depends on which.
The orbital diagram of osmium shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons the 2p subshell has 6 electrons the 3s. On the other hand the germanium atom donates two electrons in 4p orbital. The orbital diagram will be filled in the same order as described by the Aufbau principle.
1s 2 2s 2 2p 6. Electron configuration of Germanium Ge Ar 3d 10 4s 2 4p 2. What element has the following electron configuration.
The Basics of Orbital Diagrams. The orbital diagram of lead shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons the 2p subshell has 6 electrons the 3s.
Electron Configurations Ppt Download
Electron Configurations And Orbital Notation Diagrams Ppt Download

Atoms And The Periodic Table Key Terminology Activity Bundle

Solved 13 Fill In The Orbital Diagram For A Ground State Chegg Com

Structural Diversities In Heterometallic Mn Ca Cluster Chemistry From The Use Of Salicylhydroxamic Acid Mniii4ca2 Mnii Iii6ca2 Mniii Iv8ca And Mniii8ca2 Complexes With Relevance To Both High And Low Valent States Of The Oxygen Evolving
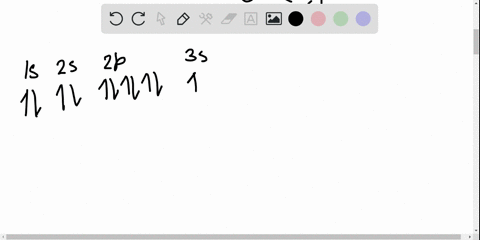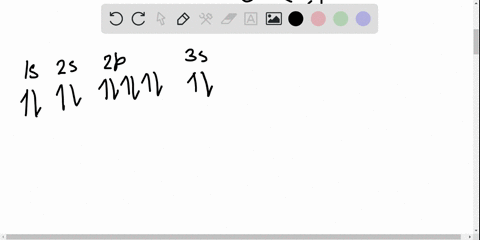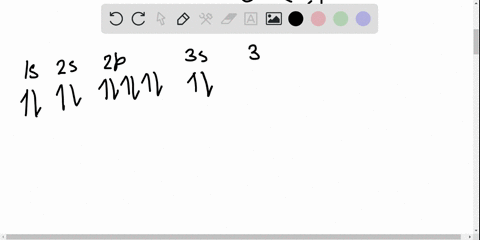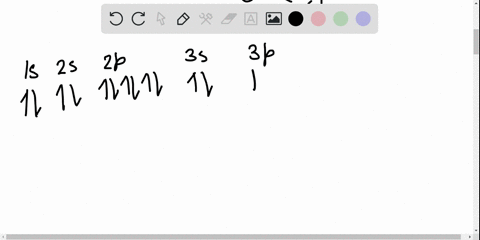
Solved Write The Orbital Diagram For The Ground State Of The Germanium Atom Give All Orbitals

Electron Configuration Tutorial Germanium Youtube
Solved Write Orbital Diagrams For The Following Atoms 15p19k28ni35br58ce Course Hero
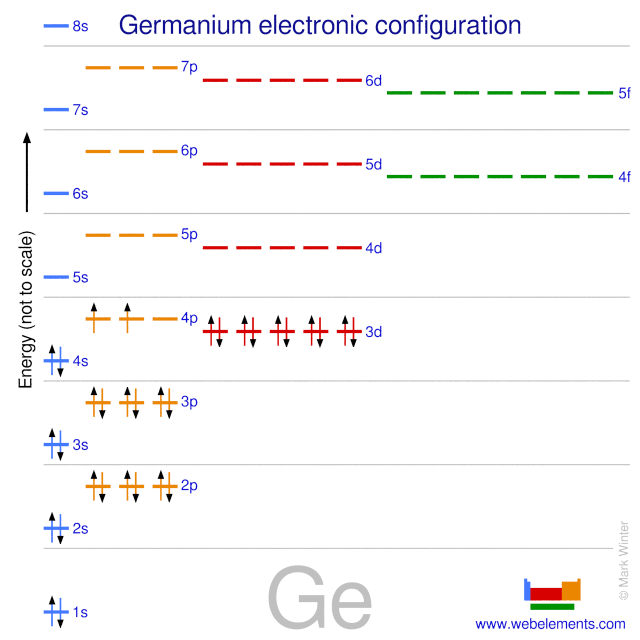
Webelements Periodic Table Germanium Properties Of Free Atoms

Polarization P Of The M 2 Line As A Function Of Beam Energy The 15 Download High Quality Scientific Diagram

Germanium Ge

Solved Write The Orbital Diagram For The Ground State Of The Germanium Atom Give All Orbitals
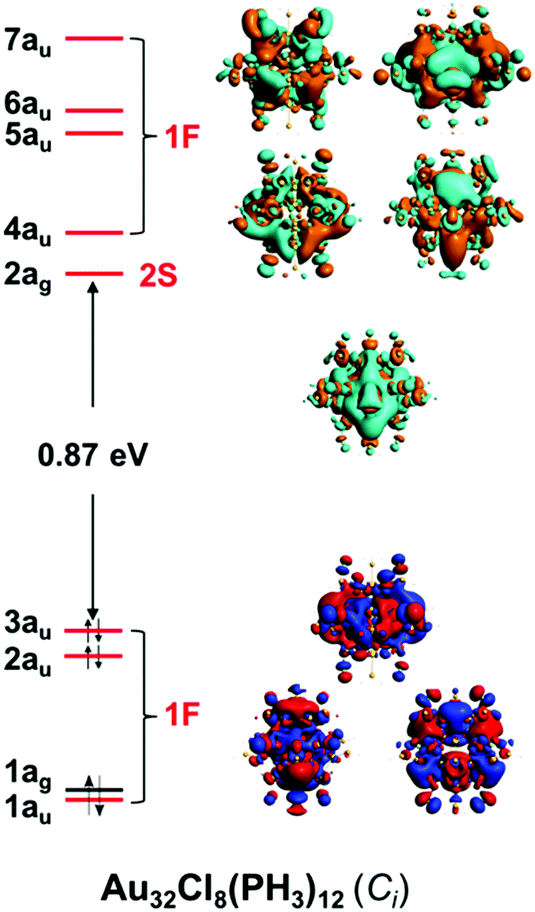
Electron Count And Electronic Structure Of Bare Icosahedral Au 32 And Au 33 Ionic Nanoclusters And Ligated Derivatives Stable Models With Intermediat Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 D0cp03735d

Germanium Ge
![]()
88 How To Write Electron Configurations And Orbital Diagrams Urdu On Vimeo
Principles Of Chem 1
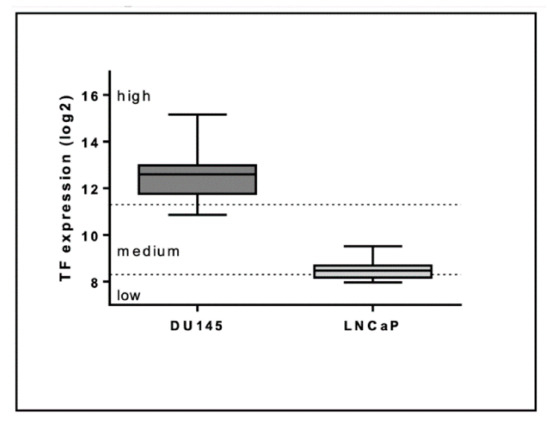
Cancers Free Full Text Extracellular Vesicle Associated Tissue Factor Activity In Prostate Cancer Patients With Disseminated Intravascular Coagulation Html